Biophysical modeling of anti-cancerous therapies: project MEMOVE
Participants: Clair Poignard, Michaël Leguèbe.
Partners:
Team of Vectorology and Anticancer Therapies at the
Institut Gustave Roussy (VAT
lab), Villejuif
Department of Mathematics of the University of Versailles
(LMV)
Bioengineering laboratory Ampère, Lyon
MEMOVE
(Multiscale Electroporation MOdeling Validated by the
Experiments) is a transdisciplinary project that aims at
developing new mathematical models, numerical tools as
well as new experimental protocols to provide a complete
understanding of the electropermeabilization from the cell
to the tissue scale. The electroporation modeling will be
developed thanks to two-way links between the numerical
simulations and the experiments: the experimental results
providing preliminary results to the modeling, which then
will highlight a priori the main phenomena of the
experiments. The fitting of the model based on PDEs is the
key point of the project. This will be performed by a data
assimilation procedure well known by the INRIA team MC2.
At the cell scale, it is proposed to develop new
electrical cell PDEs models that describe simultaneously
the electropermeabilization by micropulses, and the
electrophysiological changes of the cell during the
process (cell swelling, ions fluxes in the cell...).
Moreover a theoretical asymptotic analysis
will be performed to study the electropermeabilization of
closely
touching cells, while homogenization of single cell models
will help
in the understanding of the electroporation of cell
suspension
solution. From the experimental point of view, new device
manufacturing to simultaneously electroporate vesicles and
quantify
this electroporation will be performed. These experiments
will
highlight the specificity of cell electroporation, when
compared to
the simplest experimental cell model that is liposome.
At the tissue scale, MEMOVE aims at providing a non-linear
tissue
conductivity modeling, with parameters that will be fitted
with the
experiments on potatoes, and rat liver, through data
assimilation
techniques based on proper orthogonal decomposition
developed by
MC2. Here again, both numerical and experimental
influences of the
pulse properties on the tissue electroporation will be
investigated.
The final goal of the project, once these multiscale
models will be
derived, is to provide numerical tools that ensure the
optimal pulse
delivery for a given realistic geometry of tissue, in
order to propose
a code that could be useful to the physician applying
electrochemotherapy as cancer treatment. The full human
body meshing
developed by Ampère will be useful to perform the robust
optimization
to ensure the best pulse delivery that will be addressed
by Ampère,
MC2 and LMV. The results will be proposed to the VAT lab
to test the
procedure on the electroporation on small animals and to
evaluate the
consistence of the optimization.
O. Kavian, M. Leguèbe, C. Poignard, L. Weynans. Classical
electropermeabilization modelling at the cell scale.
Accepted in Journal of Mathematical Biology.
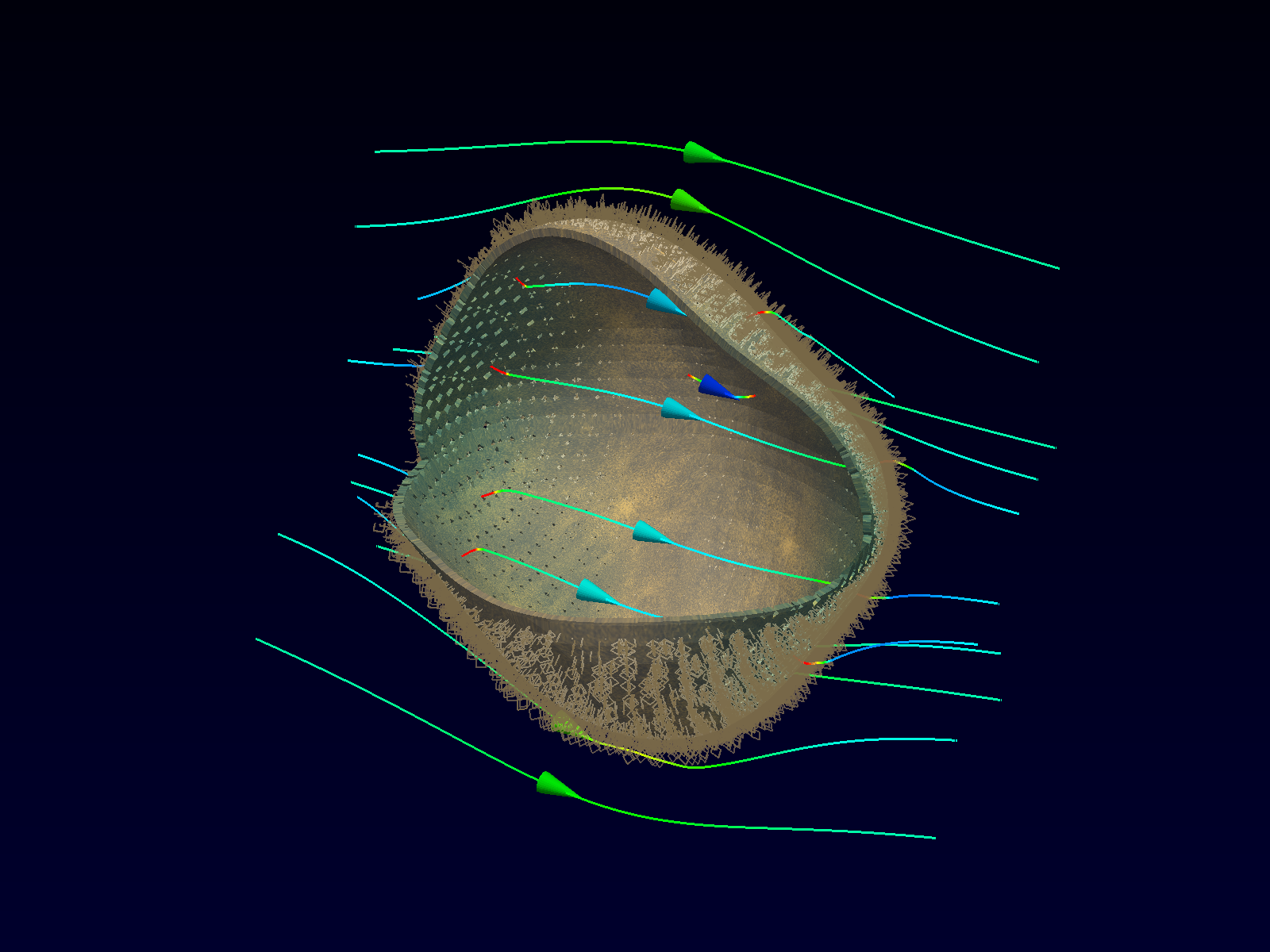
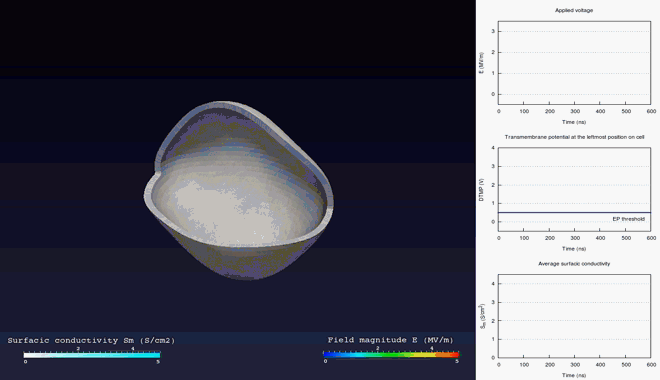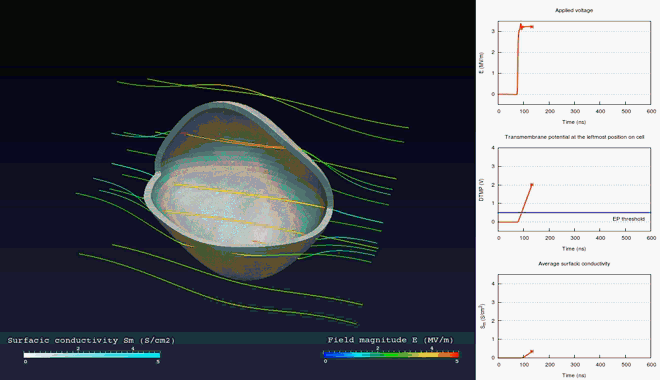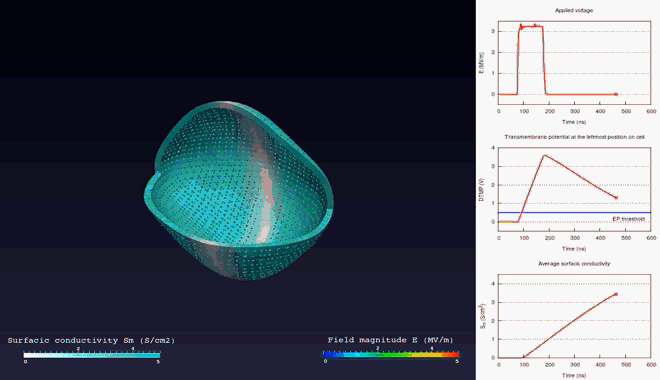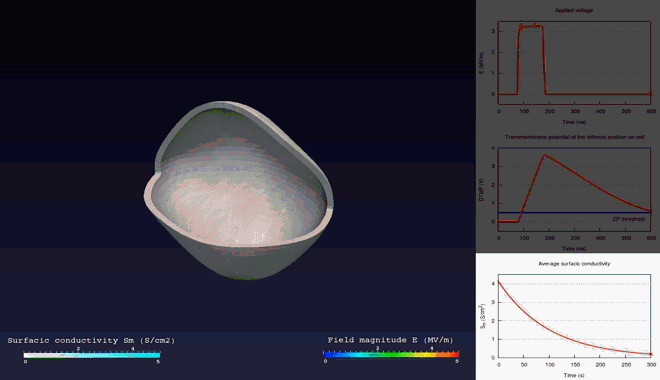
Extra- and intra-cellular electric field, with
poration in blue areas and holes of the cellular
membrane.