Theory and modeling for cancer biology
Participants: Thierry Colin, Olivier Saut, Clair Poignard, Sébastien Benzekry, Etienne Baratchart.
Biology labs involved:
Center of
Cancer Systems Biology, TUFTS University School of
Medicine, Boston, directed by Lynn Hlatky
Angiogenesis
and cancer microenvironment laboratory, Inserm,
Bordeaux, directed by the Pr Andreas Bikfalvi.
In order to gain biological understanding of complex
phenomena, we develop theoretical mathematical models for
various processes of cancer biology such as avascular and
vascular tumor growth but also development of a cancer
disease at the organism level, integrating the metastatic
process, which represents the major cause of death in a
cancer disease (90%). These models yield insights about
various topics including anti-angiogenic therapies,
metastatic dormancy or post-surgery metastatic
acceleration.
Theoretical models of tumor growth
In a series of work together with B. Ribba and E. Grenier
we have introduced a generic PDE (partial differential
equations) model for tumor growth. The models were
designed for both vascular and avascular stages. The model
is based on the description of the development of
populations of cells. We consider proliferative cells,
quiescent cells and healthy tissues. The proliferative
cells undergo a cell cycle that is regulated by various
biological processes such as hypoxia or overcrowding. The
distribution of oxygen depends on a vascular network that
is obtained through an angiogenesis model that describes
proliferation and migration of endothelial cells according
to chemotaxis phenomena regulated by secretion of several
pro- and anti-angiogenic factors (VEGF, PDGF, angiostatin,
angiopoietin,…). Interaction with the extracellular matrix
and influence of metalloproteinases are also considered.
Several mechanical aspects have been investigated
(visco-elasticity, elasticity of membranes, Darcy's law,
…). Eventually, we have also tested the influence of
several treatments (radiotherapy, chemotherapy,
anti-angiogenic drugs, inhibitors of MMP...). The model
has been implemented in a 3D framework in C++ in the
platform developed by O. Saut. More details can be found
in the following publications:
F. Lignet, S. Benzekry, S. Wilson, F. Billy, O. Saut, M.
Tod, B. You, A. Adda Berkane, S. Kassour, M.X. Wei, E.
Grenier, B. Ribba, Theoretical investigation of the
efficacy of antiangiogenic drugs combined to chemotherapy
in xenografted mice, Journal of Theoretical Biology,
Volume 320, pp. 86-99, 2013
D. Bresch, T. Colin, E. Grenier, B. Ribba, O. Saut A
viscoelastic model for avascular tumor growth, DCDS
Supplements, 101-108, Volume 2009, Issue : Special,
september 2009.
F. Billy, B. Ribba, O. Saut, H. Morre-Trouilhet, Th.
Colin, D. Bresch, J.-P. Boissel, E. Grenier, J.-P.
Flandrois, A pharmacologically-based multiscale
mathematical model of angiogenesis, and its use in
analysing the efficacy of a new anti-cancer treatment
strategy. Journal of Theoretical Biology, vol. 260, Issue
4, 21 October 2009, Pages 545-562.
Billy F., Saut O., Morre-Trouilhet H., Colin T., Bresch
D., Ribba B., Grenier E. Modèle mathématique multi-échelle
de l'angiogenèse tumorale et application à l'analyse de
l'efficacité de traitements anti-angiogéniques. Bull
Cancer, mars 2008 ; vol.95, numéro spécial : 65.
D. Bresch, T. Colin, E. Grenier, B. Ribba, O. Saut,
Computational modeling of solid tumor growth: the
avascular stage, SIAM J. SCI. COMPUT. Vol. 32, No. 4, pp.
2321–2344, 2010.
B. Ribba, Th. Colin, S. Schnell, A multi-scale
mathematical model of cancer growth and radiotherapy
efficacy: The role of cell cycle regulation in response to
irradiation, Theoretical Biology and Medical Modeling
2006, 3:7 (10 Feb 2006).
B. Ribba, O. Saut, T. Colin, D. Bresch, E. Grenier, J.P.
Boissel, A multi-scale mathematical model of avascular
tumor growth to investigate the therapeutic benefit of
anti-invasive agents, Journal of Theoretical Biology 243
(2006) 532–541.
D. Bresch, Th. Colin, E. Grenier, B. Ribba, O. Saut, O.
Singh and C. Verdier, Quelques méthodes de paramètre
d'ordre avec applications à la modélisation de processus
cancéreux, ESAIM:proc, vol. 18, 2007.
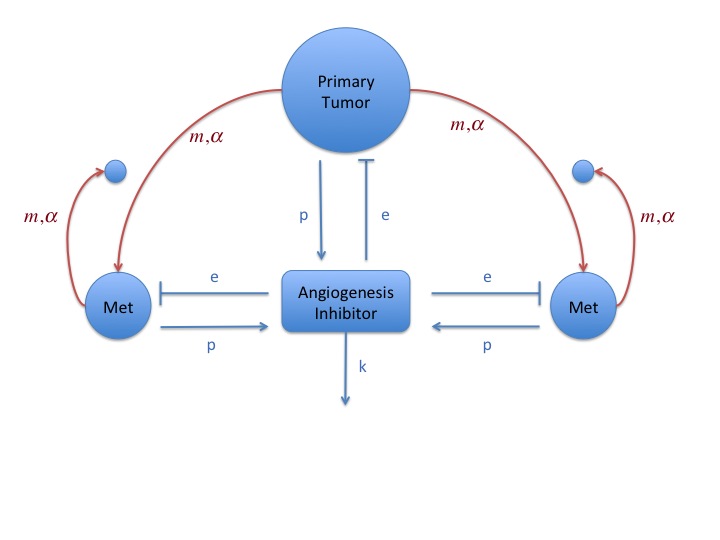
Metastatic dynamics and tumor-tumor interactions
In collaboration with the Center of Cancer and Systems
Biology in Boston (in particular with Philip Hahnfeldt),
we study angiogenic tumor-tumor interactions and the
implications for global dynamics of a cancer disease
combining biological experiments and quantitative
modeling. Indeed, tumors are known to relase in the
circulation anti-angiogenic molecules that provoke
Systemic Inhibition of Angiogenesis (SIA) and collectively
suppresses the growth of all lesions. Models are written
at the organism scale, taking into account both primary
and secondary tumors (metastases). Clinical and biological
implications of the SIA theory for metastatic global
dormancy ("Cancer without disease") and possible
acceleration of metastatic growth after removal of a
primary lesion are derived.
 |
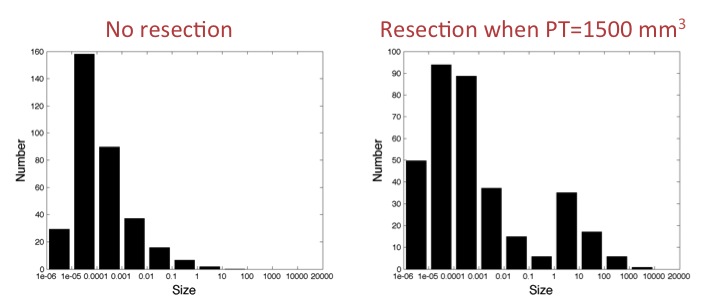 |
Model for systemic inhibition of angiogenesis and
simulation reproducing an experiment with resection or
not of primary tumor when it reaches 1500 mm3. Size
distribution of the metastases at the end shows growth
acceleration of preexisting metastases.
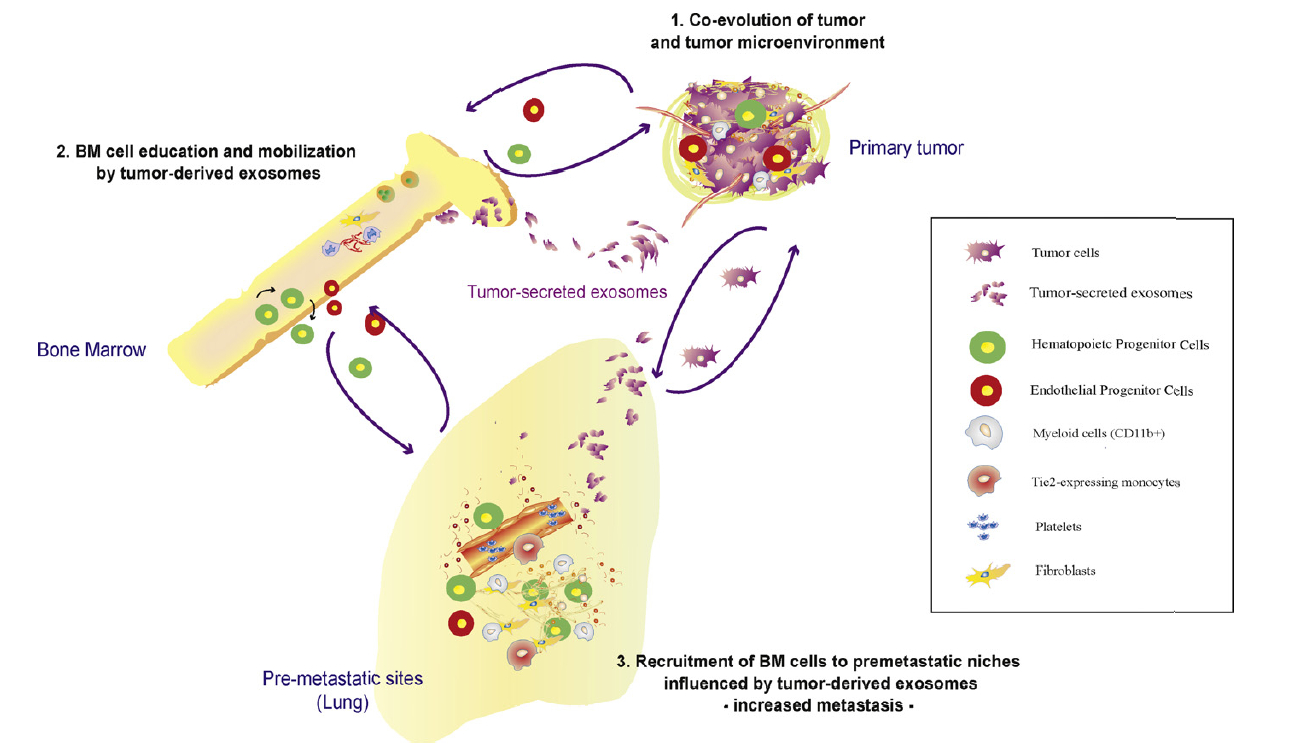
Pre-metastatic niche
Based on biological experiments performed in the
Angiogenesis and cancer microenvironment laboratory, we
try to formalize into a mathematical model the theory of
the pre-metastatic and metastatic niche. These are newly
discovered biological processes by which a pre-established
primary tumor prepares the soil in distant organs for
seeding of migratory cells that develop into metastases.
Activation of distant stroma such as fibroblast cells is
thought to be mediated by bone-marrow derived cells
recruited by cytokines emitted by the primary tumor. By
establishing and validating a mathematical model, we want
to identify critical players in this phenomenon in order
to help to develop anti-metastatic strategies.
 |
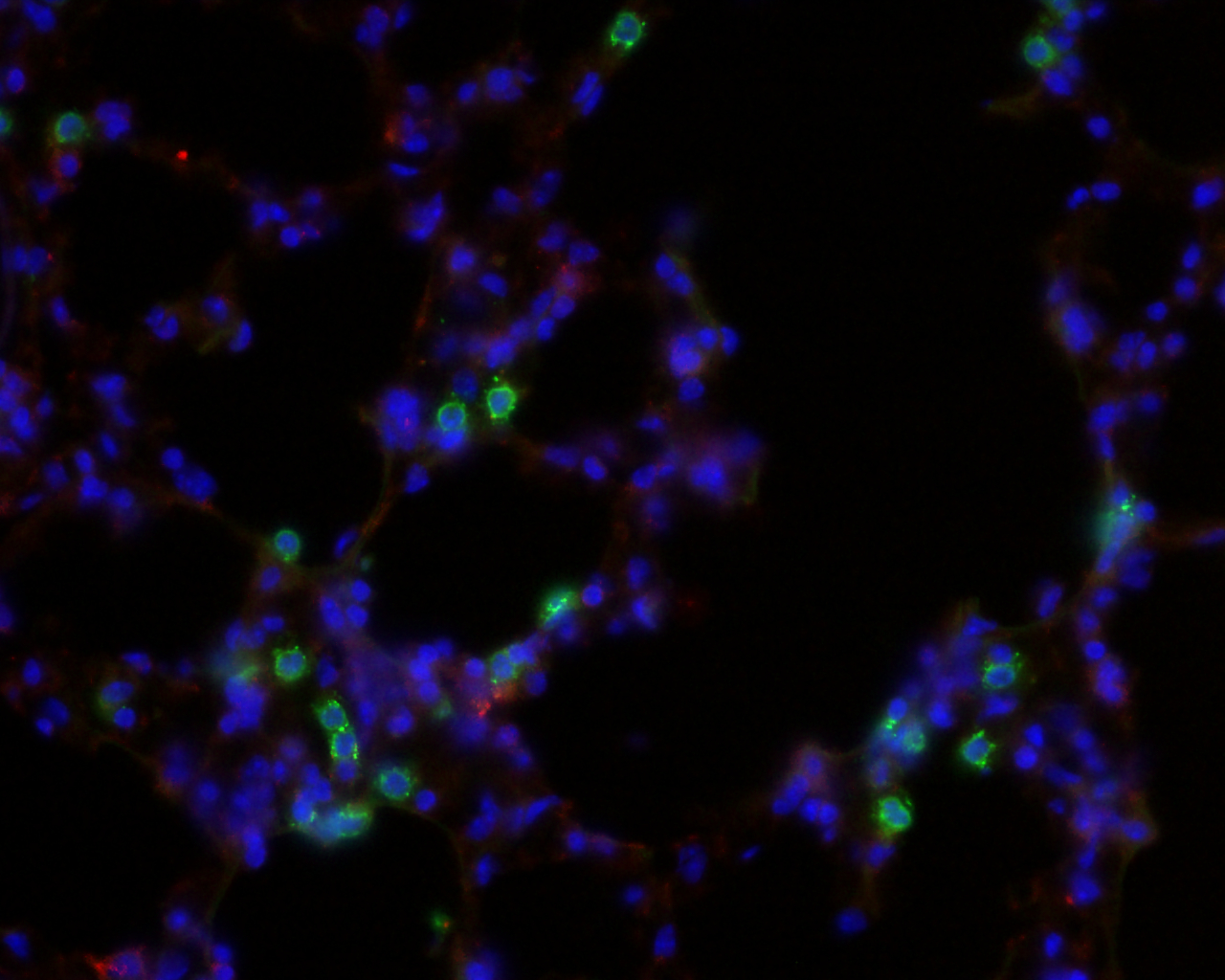 |
Left: Schematic representation of pre-metastatic nich
formation. From Peinado, Lavotshkin and Lyden, The
secreted factors responsible for pre-metastatic niche
formation: old sayings and new thoughts, Seminars in
Cancer Biology, 2011.
Right: Microscopy image of stained lung tissue.
Blue fluorescence stains for cells nuclei (DAPI) and
green fluorescence for granulocytes.